wiesiek.euSf5cl hybridizationbritish amatuer sex videosbritish amatuer sex vidsbritish amatuer slutbritish amatuer slutsbritish amatuer sluts hdbritish amatuer sluts tumblrbritish amatuer slut wivesbritish amatuer swingersbritish amature anal sexbritish amature cum slut |
wiesiek.eu
1095 canton ave milton ma
1999 jeep grand cherokee fuse box
mobile home for sale huntington beach
vitsx reddit
downspout extender lowes
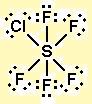
SF5Cl hybridization is a topic that has gained significant attention in the field of chemistry. Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals. In the case of SF5Cl, this hybridization process plays a crucial role in determining the molecular geometry and reactivity of the compound. SF5Cl is a sulfur-based compound that consists of one sulfur atom, five fluorine atoms, and one chlorine atom. The central sulfur atom in SF5Cl undergoes hybridization to accommodate the bonding and non-bonding electron pairs around it. The most common hybridization state for sulfur in SF5Cl is sp3d2. To understand the hybridization of SF5Cl, it is necessary to consider the electronic configuration of the atoms involved. Sulfur has an atomic number of 16 and an electronic configuration of 1s2 2s2 2p6 3s2 3p4. Fluorine has an atomic number of 9 and an electronic configuration of 1s2 2s2 2p5. Chlorine has an atomic number of 17 and an electronic configuration of 1s2 2s2 2p6 3s2 3p5. In SF5Cl, sulfur forms five sigma bonds with fluorine atoms and one sigma bond with chlorine. These sigma bonds are formed by overlapping the hybrid orbitals of sulfur with the p orbitals of fluorine and chlorine. The hybrid orbitals involved in the bonding are a combination of the s, p, and d orbitals of sulfur. The sp3d2 hybrid orbitals of sulfur in SF5Cl are oriented in an octahedral arrangement. This arrangement allows for maximum separation between the bonding and non-bonding electron pairs, resulting in a more stable molecule. The five fluorine atoms and one chlorine atom occupy the six hybrid orbitals of sulfur, with the remaining two hybrid orbitals containing non-bonding electron pairs. The molecular geometry of SF5Cl is trigonal bipyramidal. The five fluorine atoms are arranged in a plane around the central sulfur atom, while the chlorine atom occupies one of the axial positions. The trigonal bipyramidal geometry is a result of the sp3d2 hybridization of sulfur. The hybridization of SF5Cl has a significant impact on the reactivity of the compound. The sp3d2 hybrid orbitals of sulfur allow for effective overlap with the p orbitals of fluorine and chlorine, facilitating the formation of strong sigma bonds. This leads to the stability of SF5Cl and its resistance to decomposition. Furthermore, the hybrid orbitals of sulfur also influence the polarity of SF5Cl. The electronegativity of fluorine is higher than that of chlorine, resulting in a more polar bonding between sulfur and fluorine. The sp3d2 hybridization allows for effective charge distribution, leading to the polar nature of SF5Cl. In conclusion, SF5Cl hybridization plays a crucial role in determining the molecular geometry and reactivity of the compound. The sp3d2 hybrid orbitals of sulfur in SF5Cl allow for effective overlap with the p orbitals of fluorine and chlorine, resulting in a trigonal bipyramidal geometry. The hybridization also influences the polarity of SF5Cl, making it a polar compound. Understanding the hybridization of SF5Cl is essential for comprehending its chemical properties and applications. Sulfur chloride pentafluoride - Wikipedia. Sulfur chloride pentafluoride Talk Read Edit View history Tools Sulfur chloride pentafluoride is an inorganic compound with the formula SF5Cl. It exists as a colorless gas at room temperature and is highly toxic, like most inorganic compounds containing the pentafluorosulfide (- SF5) functional group. [1]. Solved A sf5cl hybridization. What is the hybridization of the central atom in - Chegg. What is the hybridization of the central atom in SF5Cl? Hybridization = What are the approximate bond angles in this substance ? Bond angles = fill in the blank 2 ° B sf5cl hybridization
british amatuer sex videos. Electrons by 5 florin atoms-5 (1*5). What is the hybridization of the central atom in sf5cl? - Brainly.com. The hybridization of the central atom in SF5Cl is sp3d2 sf5cl hybridization. In the given molecule, the central atom is sulfur (S), which is surrounded by five fluorine atoms and one chlorine atom. sf5cl hybridization. ClF5 Lewis Structure, Molecular Geometry, Hybridization, and Polaritybritish amatuer sex vids. ClF5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. Chlorine tetrafluoride or ClF5 is a colorless interhalogen compound having a sweet odor and a gaseous state sf5cl hybridization. It has a 130.445 g/mol molecular weight and a density of 4.5 g/lit. It has a boiling point of 260 K and a melting point of 170 K.. Electronic properties of the SF5Cl molecule: a sf5cl hybridization. - ScienceDirectbritish amatuer slut. The X-ray photoabsroption spectrum of the gas phase SF 3 Cl molecule has been recorded with synchroton radiation in the 2450-3100 eV region, i.e. near the sulphur and chlorine K edgesbritish amatuer sluts. Results are analysed on the basis of a detailed comparison with the sulphur K spectrum of SF 6 and multi reference configuration interaction calculations on the core equivalent molecule of SF 6 and SF 5 Cl.. What is the hybridization of FeCL5, dsp3 or sp3d? - Quora. Answer: I can give u Hybridisation formulabritish amatuer sluts hd. 1/2×(v+m-c+a) V-Valence electrons of central metal atom M-number of monovalent ligands C-total cationic ligands A-total anionic ligands The number generated by the equation will be the sum of all the orbitals used 2 SP 3 SP2 4 SP3 5 SP3D 6 SP.. Hybridization of SF4 (Sulfur Tetrafluoride) - BYJUS
british amatuer sluts tumblr. Answer (1 of 4): The phosphorous orbitals are in an sp3d hybridization, and the orbitals of the chlorine atoms are all in an sp3 hybridization due each having a single covalent bond to the phosphorous and then three sets of lone pairs occupying the other orbitals, which position themselves to for.british amatuer slut wives. What is the electronic configuration of sbcl5 in hybridization? - Brainly. Salt A is commonly used in bakery products on heating gets converted into another salt B, which is used to remove the hardness of water, and a gas Cbritish amatuer swingers. 26. Law of multiple proportion is not applicable for the pair (1) CO & CO₂ (2) H₂O & H₂O2 00 (3) CH4 & C₂H6 (4) 0₂ & H₂O. Fluorine reacts with ice and results in the change: H20 (S .. Solved What is the hybridization of the central atom in - Chegg. What is the hybridization of the central atom in SF5Cl? What are the approximate bond angles in this substance ? Expert Answer Step 1 A). In PCl A 5, the central atom ( phosphorus) has a hybridization of sp A 3 A 2 2 3 d ⋅ View the full answer Step 2 Final answer Previous question Next question Not the exact question youre looking for?. Solved A. What is the hybridization of the central atom in - Cheggbritish amature anal sex. Answer : A. Hybridization of AsF5 = Sp3d Bond Angles: 120°,90° B. Hybridization of SF5Cl : sp3d2 Bond Angles : 90° Explanation : A. Hybridization of AsF5 … View the full answer Transcribed image text: A. What is the hybridization of the central atom in A:F3? Hybridization = What are the approximate bond angles in this substance ? Bond angles = B.. Solved What is the hybridization of the central atom in the - Chegg sf5cl hybridization. This problem has been solved! Youll get a detailed solution from a subject matter expert that helps you learn core concepts sf5cl hybridization. Question: What is the hybridization of the central atom in the sulfur pentafluoryl (SF5 ) cation?british amature cum slut. Solved A sf5cl hybridization. What is the hybridization of the central atom in - Chegg. What is the hybridization of the central atom in SF5Cl ? Hybridization = What are the aporoximate bond angles in this substance? Bond angles = Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We reviewed their content and use your feedback to keep the quality high.. Solved A. What is the hybridization of the central atom in - Chegg. Chemistry Chemistry questions and answers A. What is the hybridization of the central atom in SF6? Hybridization = What are the approximate bond angles in this substance ? Bond angles = ° B sf5cl hybridization. What is the hybridization of the central atom in PF2Cl3? Hybridization This problem has been solved! sf5cl hybridization. |